The aim of the EPCS III project is to objectify the benefits of minimally invasive interventional pain management techniques in patients with vertebral algic syndrome, and to quantify long-term pain relief and improve patients’ quality of life after disc FX on the intervertebral disc in a selected group of patients after meeting the inclusion criteria.
The study was approved by the Regional Ethics Committee on 23 April 2015 under the EC number: 9N-2015. Subsequently, it was registered in the international database of the National Health Service USA https://clinicaltrials.gov PRS: NCT02461654
EPCS 3 Approval document.
The project meets all the attributes and valid legislative standards related to medical performance approved by the Ministries of Health in the Slovak Republic and the Czech Republic.
Study detail
The EuroPainClinics®Study III study (EPCS III) is focused on clinical research of the Disc FX treatment method.
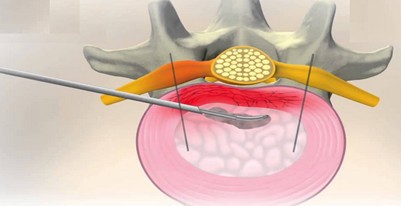
Disc FX – indication criteria
Disc FX is an innovative system enabling safe and efficient access to the affected intervertebral disc without damaging the surrounding structures. This method provides an opportunity for those who have not been successful with conservative treatment and are not yet ready for a complicated surgery. It is a minimally invasive procedure performed on an outpatient basis. The realization of the procedure consists of approaching the intervertebral disc with a special needle, which serves as a working channel. A portion of the degenerate inner tissue is removed through this needle. The disc is then sealed with a radio frequency probe to reduce the risk of repeated herniation.
Finally, the pathological nerves in the back of the disc are removed by radiofrequency. The EPCS III study aims to monitor long-term pain relief and improve quality of life in a selected group of patients who underwent a minimally invasive procedure of an intervertebral disc FX.
Subject of the EPCS III study and registration
The subject of the study is the realization of a multicenter prospective observational clinical study assessing the comparison of the development of the patient’s clinical condition in 3 time periods: before the operation, after 6 months after the operation and 12 months after the operation.
The study was prepared by Europainclinics. After elaborating the study design, preparation of the informed consents for its participants and preparation of the protocol, the study was approved on 23 April 2015 by the Regional Ethics Committee under the EC number: 9N-2015. It was subsequently registered in the international database of the National Health Service USA https://clinicaltrials.gov PRS: NCT02461654.
The project meets all the attributes and valid legislative standards related to medical performance approved by the Ministries of Health in the Slovak Republic and the Czech Republic. The processing of patients’ personal data for the purpose of conducting the study is in accordance with the current version of the 2008 Helsinki Declaration and in accordance with the relevant laws of the country. The protection of the patient’s personal data will be maintained. The data will be handled according to EU directives: Directive 95/46 / EC, Directive 2002/58 / EC, Directive 2006/24 / EC. It will be ensured that the study protocol, informed consents to the intervention, and the ongoing study are provided to the appropriate independent ethics committee according to local requirements. If required by the laws of the country, EuroPainClinics is responsible for providing the annual documentation update to an independent ethics committee. The results obtained in the study will be published in professional international journals and taken into account in the recommendations and procedures in the clinical practice of interventional pain management.
Objectives of the EPCS III study and monitored parameters
The aim of the project is to objectify the benefits of minimally invasive interventional pain management techniques in patients with vertebrogenic algic syndrome and to quantify long-term pain relief and improve the quality of life of patients after performing Disc FX. The obtained results will be compared with the results of other interventional pain management workplaces in the world.
From the monitored parameters, the clinical condition, visual pain scale, global pain score, spread of pain in the respective dermatomes, reduction of analgesic consumption, Oswestry disability questionnaire (indicating the quality of life in patients with lumbosacral pain) will be compared. The monitored parameters will be recorded in three time periods: before the operation (first examination), 6 months after the operation (second examination) and finally 12 months after the operation (third examination). The results will then be subjected to statistical analysis. Another goal of the project is to publish the results in professional international journals.
