The prospective observational comparative study EuroPainClinics®Study V (EPCS V) is focused on clinical research of the treatment method endoscopic discectomy. It compares changes in neurological status, evaluation of algesiological scales in patients who undergo this intervention after meeting the inclusion criteria. The study is approved by the ethics committee and its detailed description is available in the international database www.clinicaltrials.gov, where the study is registered under the unique identifier PRS: NCT01541163.
Description of the issue
Back pain is one of the most common forms of chronic pains, which is a multi-etiological problem in terms of diagnosis. Its most common cause is disruption of the anatomical structures of the spine, as well as functional changes, in which pain is the dominant symptom. Herniation of the intervertebral disc, with a prevalence of 1-3% in developed countries, is the most common reason leading to spinal surgery.
Endoscopic discectomy: indication criteria
Selection of patients for the study:
Patients who undergo minimally invasive interventional endoscopic discectomy after meeting all indication criteria will be included in the study: unilateral radiation of lower limb pain, corresponding to MRI findings, positive Lasegue test, insufficient effect of current fully utilized conservative treatment, positive MRI finding on the lumbosacral spine (herniation of the intervertebral disc, protrusion of the disc, free sequester), patient consent. Exclusion criteria: significant spinal canal stenosis or segmental instability on the MRI image, Cauda equina syndrome, extreme lateral disc herniation, infection, trauma, fracture.
Treatment description
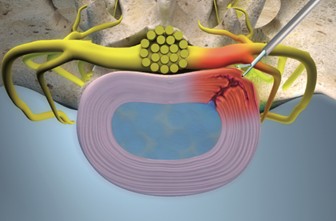
Endoscopic discectomy is an alternative to the open surgical approach – hemilaminectomy of the lumbar spine. A transforaminal or interlaminar approach is possible. It belongs to the minimally invasive endoscopic techniques, which have been successfully utilised during the treatment of degenerative intervertebral disc diseases. Due to the anatomical proportions, it is possible to perform this medical procedure in the whole area of the lumbar spine L1-L5 and in the area of the lumbosacral spine L5-S1. The procedure is performed through a 1cm incision used as a working channel to minimise the surrounding tissue trauma. The transforminal approach to the herniation enables us to completely omit the damage of spinal cord layers, which is most probably a key factor in the prevention of the formation of epidural fibrosis. Most recent evidence shows that endoscopic discectomy compared to other open approaches leads to a significantly lower blood loss, showert hospitalisation stay and a higher patient satisfaction rate, while it does not show worse results in terms of re-operations or other complications.
Subject and registration of EPCS V
Patients who undergo a minimally invasive interventional endoscopic discectomy after meeting all indication criteria will be included in the study. The study will last 36 months. The study is approved by the ethics committee. It will take place at EuroPainClinics® workplaces in Prague, Bratislava, Bardejov and in Košice. All procedures are standard procedures used in the treatment of back pain and they have been approved by the Ministry of Health of the Slovak republic and Czech republic. The detailed description of the study is available in the international database www.clinicaltrials.gov, where the study is registered under the unique identifier PRS: NCT01541163
Goals and parameters of EPCS V
Study blinding, performance implementation, data collection and processing:
Patients will be divided into two groups based on the performed procedure, which does not affect the inclusion into the study. Based on further clinical examinations in indicated cases, a minimally-invasive intervention will be proposed for the patient. A patient meeting the inclusion criteria of the study will be informed by the treating physician about the study performed and will be offered the opportunity to participate. In the case of a patient’s consent, they will be informed in detail about the intervention of Endoscopic discectomy, and will then sign the informed consent for the participation in the study and the informed consent for a medical minimally invasive procedure – endoscopic discectomy. The patient will be assigned a number generated by a random number generator. After completing the first pre-procedure report, the report will be sent to the study coordinator and the person in charge of processing the study data. The first and second postoperative examination of the patient will be performed by another doctor (not performing the procedure itself), at another interventional pain management center and the clinical reports will be filled in again. During post-operative follow-up examinations, the examining physician will be informed of the patient’s participation in the study at the outpatient visit. The patient is identified by the assigned generated number. The physician will perform a clinical examination of the patient without knowing which procedure the patient underwent and will complete the study protocol, which they will send to the person in charge of processing the study data.
The first postoperative follow-up examination will be provided by the study coordinator after 6 months and the second postoperative follow-up examination after 12 months.
